A.
21 CFR Part 11 is a U.S. FDA regulation that specifies requirements for electronic records and electronic signatures, with broad impact across regulated industries.
[Configuration Steps]
1. In TDS, click [Project] - [Security] from the top menu.
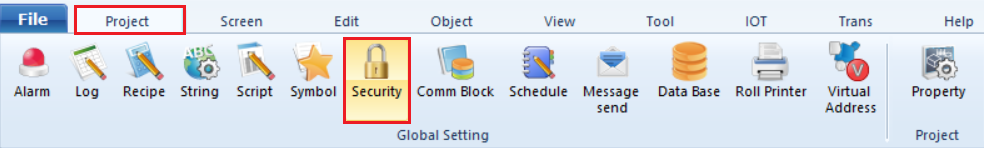
2. In the Security Settings window, click [21 CFR Part11 Option].
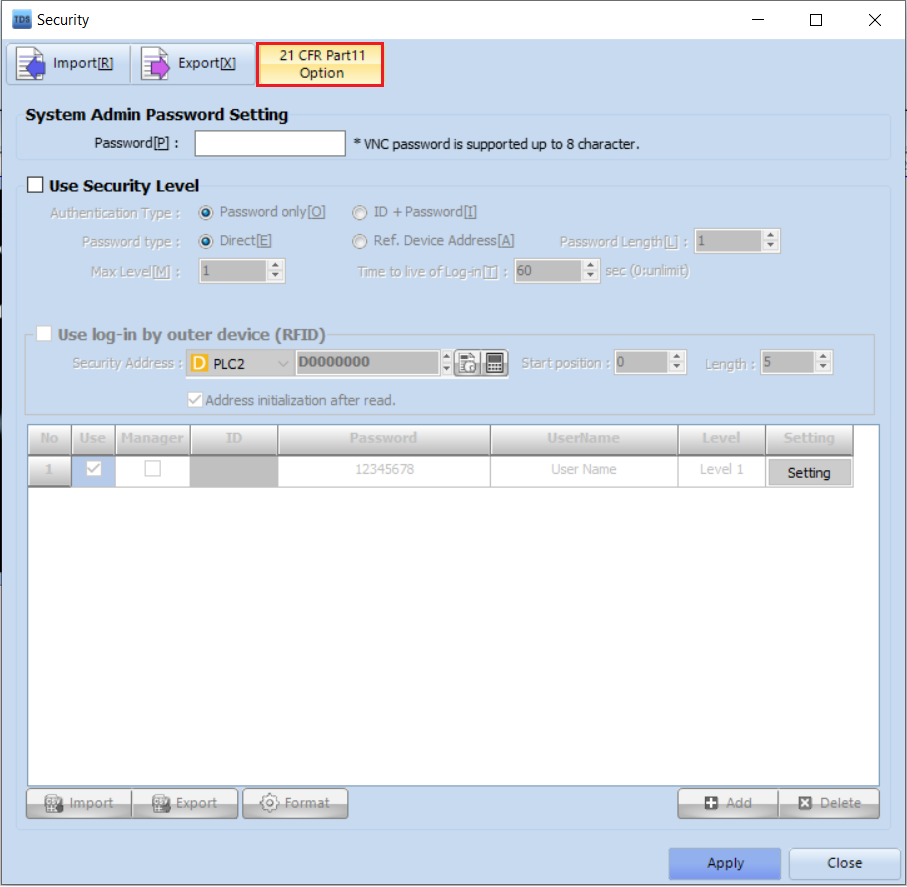
3. Select the items you wish to apply, and the changes will be saved automatically.
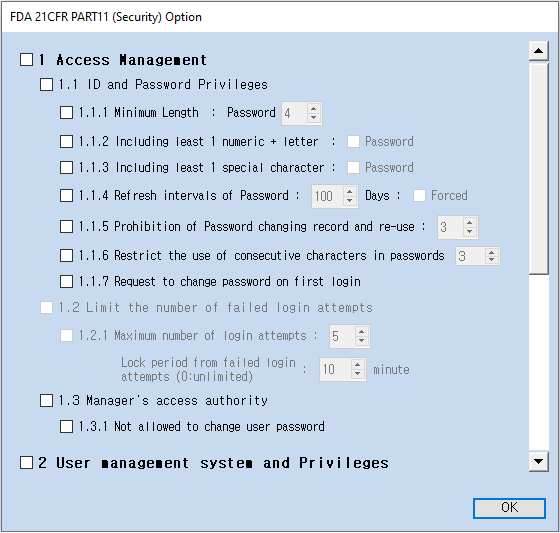
※ Note: For detailed explanations of each option, refer to [Chapter 4.7.4 – 21 CFR Part 11 Option] in the [TOP DESIGN STUDIO User's Manual].